The experiments were made using varius standard AA alcaline battery .This type of battery have a capacity in the range of 2 Amp.hour depending in a large extent of the discharge current .
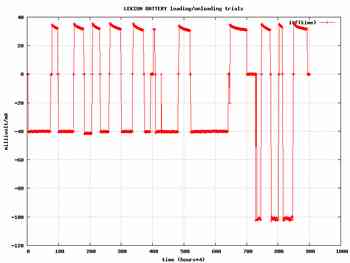
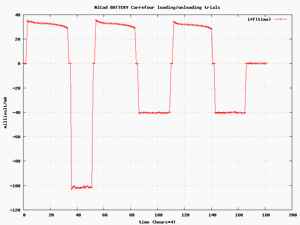
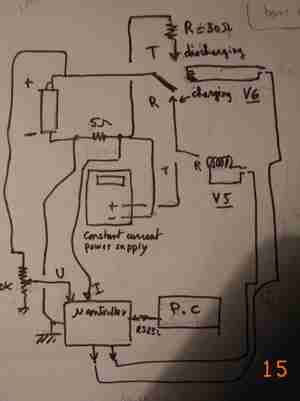
The set up used for the test consist of a microcontroller switching the battery either to a constant current power supply for loading or to a resistor for the discharge .The voltage across the battery is measured as the current flowing in the battery .These data , measured with a 12 bit A/N circuit every 15 minutes are stored , thanks to a PC collecting them for further use .A typical cycle is as followed :
* settling time of 30 mn with no current to measure voltage
* charging at constant current during X hours
* settling time of 30 mn with no current to measure voltage
* discharge down to a given voltage across the battery
* settling time of 30 mn with no current to measure voltage
The process is repeated as many times as desired .
As stated earlier the capacity of a battery depend on a large extend of the discharge rate .This discharge rate an be quite different from a battery feeding a clock or for a battery feeding a radio transmitter with a lot of cases in between .In our own case the discharge current is generaly in the range of 20 to 40 mA and all experiments were dons in that range
The data collected by the PC were plotted using GNUPLOT a versatile ploting program

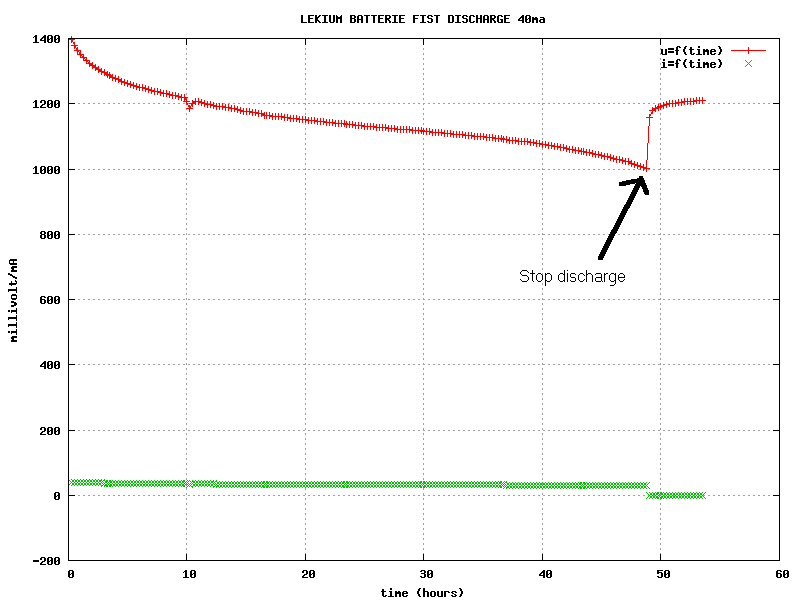
 volt .It should
be noted that the capacity is only 1.28 amp.hour if the final voltage
is 1.100 volts .The final resistance of the battery at the end of the
discharge (R=Delta U/Delta I) was found to be equal to 6.2
Ohm .
volt .It should
be noted that the capacity is only 1.28 amp.hour if the final voltage
is 1.100 volts .The final resistance of the battery at the end of the
discharge (R=Delta U/Delta I) was found to be equal to 6.2
Ohm .