 Example 1Adding
10g of methyl dehydrojasmonate (Compound 1) to a reaction vessel
containing 0.05g of 5wt% Pt/C and 10ml of methanol, substituting with
nitrogen three times, charging 1MPa of hydrogen, stirring at 50 ℃ until
the reaction of the raw materials is completed, and stopping the
reaction. To obtain a reaction solution containing methyl
3-hydroxy-2-pentyl-cyclopentenylacetate (compound 2).And
(3) reducing the reaction temperature of the reaction liquid to 10 ℃,
adjusting the hydrogen pressure to 2MPa, stirring until the raw
materials are reacted, and stopping the reaction. Filtering with celite
pad, adding saturated sodium bicarbonate into the filtrate, extracting,
rectifying under reduced pressure, and separating to obtain colorless
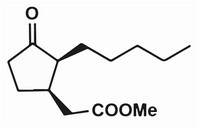
oily cis-3-hydroxy-2-pentyl-cyclopentyl methyl acetate (compound 3)
with yield of 95%. H-NMR (400MHz, CDCl3): 0.89 (t, J =7.0,3H);
1.12-1.20 (m, 1H); 1.22-1.38 (m, 7H); 1.47-1.58 (m, 1H); 1.77-1.83 (m,
2H); 1.91-1.98 (m, 1H); 2.02-2.10 (m, 2H); 2.14 (dd, J =10.0,14.6,1H);
2.38 (dd, J =6.2,14.6,1H); 2.58-2.67 (m, 1H); 3.67 (s, 3H); 3.99 (dt, J
=4.4,6.8,1H).8.0g
of cis-3-hydroxy-2-pentyl-cyclopentylacetic acid methyl ester (compound
3) was dissolved in 10ml of dichloromethane, 0.1wt% of cesium carbonate
was added, the mixture was opened to the atmosphere, the mixture was
stirred at 25 ℃ until the reaction was completed, and the reaction was
stopped after completion of the reaction of the starting materials was
detected. Extraction and reduced pressure distillation are carried out,
thus obtaining the cis-methyl dihydrojasmonate (compound 4) of
colorless oil, the yield is 90 percent, and the cis-isomer content is
92 percent. H-NMR (400MHz, CDCl3): 0.89 (t, J =7.0Hz, 3H); 1.54-1.11
(m, 6H); 1.58 (m, 1H); 1.86 (m, 1H); 2.27-2.07 (m, 5H); 2.30 (q, J
=7.2hz, 1h); 2.42 (dd, J =5.4,15.6hz, 1h); 2.82 (m, 1H); 3.66 (s, 3H).
Example 1Adding
10g of methyl dehydrojasmonate (Compound 1) to a reaction vessel
containing 0.05g of 5wt% Pt/C and 10ml of methanol, substituting with
nitrogen three times, charging 1MPa of hydrogen, stirring at 50 ℃ until
the reaction of the raw materials is completed, and stopping the
reaction. To obtain a reaction solution containing methyl
3-hydroxy-2-pentyl-cyclopentenylacetate (compound 2).And
(3) reducing the reaction temperature of the reaction liquid to 10 ℃,
adjusting the hydrogen pressure to 2MPa, stirring until the raw
materials are reacted, and stopping the reaction. Filtering with celite
pad, adding saturated sodium bicarbonate into the filtrate, extracting,
rectifying under reduced pressure, and separating to obtain colorless
oily cis-3-hydroxy-2-pentyl-cyclopentyl methyl acetate (compound 3)
with yield of 95%. H-NMR (400MHz, CDCl3): 0.89 (t, J =7.0,3H);
1.12-1.20 (m, 1H); 1.22-1.38 (m, 7H); 1.47-1.58 (m, 1H); 1.77-1.83 (m,
2H); 1.91-1.98 (m, 1H); 2.02-2.10 (m, 2H); 2.14 (dd, J =10.0,14.6,1H);
2.38 (dd, J =6.2,14.6,1H); 2.58-2.67 (m, 1H); 3.67 (s, 3H); 3.99 (dt, J
=4.4,6.8,1H).8.0g
of cis-3-hydroxy-2-pentyl-cyclopentylacetic acid methyl ester (compound
3) was dissolved in 10ml of dichloromethane, 0.1wt% of cesium carbonate
was added, the mixture was opened to the atmosphere, the mixture was
stirred at 25 ℃ until the reaction was completed, and the reaction was
stopped after completion of the reaction of the starting materials was
detected. Extraction and reduced pressure distillation are carried out,
thus obtaining the cis-methyl dihydrojasmonate (compound 4) of
colorless oil, the yield is 90 percent, and the cis-isomer content is
92 percent. H-NMR (400MHz, CDCl3): 0.89 (t, J =7.0Hz, 3H); 1.54-1.11
(m, 6H); 1.58 (m, 1H); 1.86 (m, 1H); 2.27-2.07 (m, 5H); 2.30 (q, J
=7.2hz, 1h); 2.42 (dd, J =5.4,15.6hz, 1h); 2.82 (m, 1H); 3.66 (s, 3H).
Publication number
Priority date
Publication date
Assignee
Title
JPH0665151A
*
1992-08-25
1994-03-08
Japan Tobacco Inc
Production of dihydroepijasmic acid methyl ester
WO2004108652A2
*
2003-06-06
2004-12-16
Universita' Degli Studi Di Pavia
Enantioselective process for the preparation of methyl dihydroepijasmonate
CN100999466A
*
2006-01-11
2007-07-18
浙江新和成股份有限公司
Synthesizing process of cis-dihydro jasmine keto-acid methyl ester
CN101475480A
*
2008-12-11
2009-07-08
淮安万邦香料工业有限公司
Method for synthesizing cis-methyl dihydrojasmonate
CN101613277A
*
2009-07-30
2009-12-30
淮阴师范学院
Improve the method for content of cis-methyl-dihydrojasmonate
CN101914000A
*
2010-08-18
2010-12-15
北京航空航天大学
Method for preparing aldehyde or ketone by non-catalytic reaction
CN108863787A
*
2017-05-12
2018-11-23
南开大学
3- alkyl
-2- carbethoxyl group substituted cyclic is conjugated asymmetric
catalytic hydrogenation and its application of ketenes
CN109384675A
*
2017-08-14
2019-02-26
南开大学
The Enantioselective total synthesis method of needle juniper celery alkane type diterpene and the like
**********************************************************************************************************
Method for preparing methyl dihydrojasmonate CN
CN101519355B 胡雨来 西北师范大学
Priority 2009-03-17 • Filed 2009-03-17 • Granted 2011-11-30 • Published 2011-11-30
Abstract
The
invention provides a method for synthesizing methyl dihydrojasmonate.
2-pentyl cyclopentenone is adopted as a raw material; the raw material
and allyl zinc bromide are subjected to 1,4-Michael addition to form
2-pentyl-3-allyl cyclopentanone; the 2-pentyl-3-allyl cyclopentanone is
oxidized by sodium periodate in the presence of a ruthenium trichloride
hexahydrate catalyst to synthesize dihydrojasmonic acid; and then the
dihydrojasmonic acid is esterified to form the methyl dihydrojasmonate.
The raw material of the method is cheap and low in cost; the process is
simple and convenient in operation; the reaction conditions are mild
and easy to control; the synthesis route is short and the synthesis
period is reduced greatly; and the reaction product is single, high in
yield (up to 65 to 85 percent), less in environmental pollution, and
environmentally-friendly.
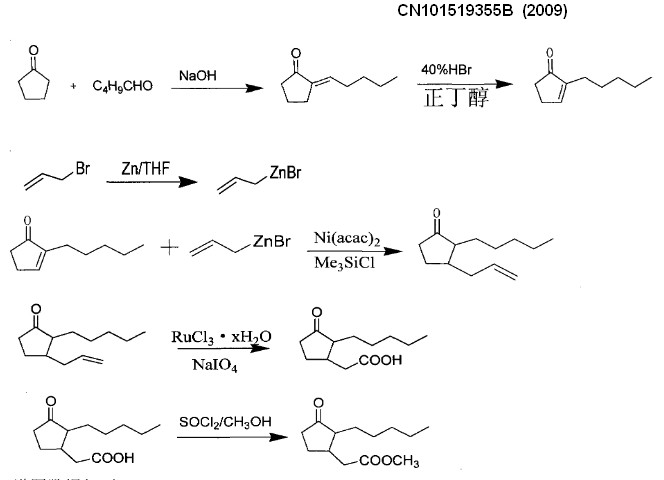
Embodiment
The present invention is described further below by concrete enforcement.
Step 1:
the preparation of 2-amyl cyclopentenoneThe 2-amyl cyclopentenone can be bought from the market, also can get by following prepared:
With
NaOH (0.83g), H 2O (75ml) adds there-necked flask, is heated to 25 ℃
with water-bath, drips cyclopentanone (38g) from dropping funnel, keeps
temperature of reaction to be no more than 32 ℃ during dropping, drips
valeraldehyde (22g) after adding again, keeps reacting liquid
temperature to be no more than 32 ℃.Add the back at 28 ℃ of reaction
1h, add acetate (1.5g) and stir 2min, tell organic layer, once with the
saturated common salt washing.
Add NaHSO 4Solution at room
temperature stirs 20min, adds a small amount of propyl carbinol.Tell
organic layer, wash once with saturated common salt aqueous
solution.Organic layer is put into round-bottomed flask, add the HBr
(6.5ml) of propyl carbinol (132ml) and 40%, behind the reflux 1.5h,,
pour separating funnel into, use saturated NaCl, NaHCO successively the
reaction solution cooling 3, the NaCl solution washing, the anhydrous
MgSO of organic layer 4Dry.With water pump pressure reducing and
steaming propyl carbinol, use the oil pump underpressure distillation
again.Collect 130 ℃~140 ℃, the cut of 0.085MPa gets colourless liquid,
is the 2-amyl cyclopentenone.Productive rate 65%.Its reaction formula
is as follows:
Figure G2009100218015D00031
The spectral data of product characterizes as follows:
IR(KBr)v maxcm -1:2926,2858,1704,1633;
1HNMR(400MHz,CDCl 3):δ=0.83-0.90(m,3H),1.25-2.58(m,12H),7.29-7.31(m,1H);
13CNMR(100Hz,CDCl 3):δ=210.1,157.3,146.5,34.5,31.5,27.4,26.4,22.4,13.9。
Step 2:
the preparation of 2-amyl group-3-allyl group cyclopentanone(1) preparation of allyl group bromination zincon
Allyl group bromination zincon can be bought from the market, also can get by following prepared:
In
exsiccant two neck bottles (50ml), put into zinc powder (0.021mol),,
add tetrahydrofuran (THF) (THF) (3ml) with 1 with the air in the
nitrogen replacement bottle, 2-ethylene dibromide (4~5), the reaction
mixture heating up to there being bubble to produce, is naturally
cooled to room temperature, add trimethylchlorosilane (Me 3SiCl) (4~5),
stirring at room 15min adds allyl bromide 98 (0.020mol) and
tetrahydrofuran (THF) (THF) (10ml) then, and stirring reaction is
standby.Its reaction formula is as follows:
Figure G2009100218015D00041
(2)
under nitrogen protection, in another 100ml three-necked bottle, put
into acetylacetonate nickel (Ni (acac) 2) (0.0012mol), triethylamine
(Et 3N) or triphenyl phosphorus (Ph 3P) (0.0048mol), tetrahydrofuran
(THF) (THF) (10ml) is heated to reaction mixture 60 ℃, and stirring
reaction 10min is cooled to room temperature, slowly adds the allyl
group bromination zincon of above-mentioned preparation, after adding
reaction mixture is chilled to-15 ℃ with the cryosel bath.From dropping
funnel, drip 2-amyl cyclopentenone (0.010mol), trimethylchlorosilane
(Me then 3SiCl) (0.018mol) and the mixture (5ml) formed of
tetrahydrofuran (THF) (THF).Add the back and continue stirring
reaction, allow cryosel bathe nature and be warming up to room
temperature, coreaction 12 hours.The 5mol/L HCl that in reaction flask,
adds 10ml then, restir reaction 1 hour.Add the 10ml ether, organic
phase is told in extraction.Use anhydrous magnesium sulfate drying,
residuum carried out column chromatography after boiling off solvent,
colourless or light yellow liquid, be 2-amyl group-3-allyl group
cyclopentanone.Productive rate 73%.Its reaction formula is as follows:
The spectral data of product characterizes as follows:
IR(KBr)v maxcm -1:2927,2858,1740,1641;
1HNMR(400MHz,CDCl 3):δ=0.87(t,J=7.0Hz,3H),1.26-2.43(m,16H),5.05-5.10(m,1H),5.77-5.79(m,1H),5.81-5.88(m,1H);
13CNMR(100Hz,CDCl 3):δ=221.1,135.8,116.6,54.2,41.1,38.6,37.7,32.1,28.0,26.5,26.4,22.4,14.0。
S
tep 3: the preparation of Dihydrojasmone acidIn
the 50ml round-bottomed flask, add 0.388g 2-amyl group-3-allyl group
cyclopentenone, add 7ml water and 7ml acetonitrile again, add the 1g
sodium periodate then, stir, add the 0.02g hydrate ruthenium
trichloride again, the temperature of solution rises to 30 ℃ gradually,
keep temperature of charge under 12 ℃~20 ℃, slowly add the 3.5g sodium
periodate, reactant was stirred 2 hours down at 17 ℃, added the ethyl
acetate stirring reaction then 1 hour, filter, filtrate is used
saturated Na then with 0.1mol/L salt acid elution 2S 2O 3Solution
washing is used ethyl acetate extraction, collected organic layer, and
solvent evaporated gets yellow pasty state liquid, and column
chromatography gets colourless liquid, is Dihydrojasmone
acid.Productive rate 92%.Its reaction formula is as follows:
Figure G2009100218015D00051
The production spectra diagram data characterizes as follows:
IR(KBr)v maxcm -1:2924,2854,1739,1710,1459;
1HNMR(400MHz,CDCl 3):δ=0.88(t,J=7.2Hz,3H),1.23-2.68(m,16H),11.18(s,1H);
13CNMR(100Hz,CDCl 3):δ=219.6,178.1,54.1,38.7,37.7,37.6,32.0,27.7,27.1,26.2,22.4,14.0。
Step 4: the preparation of methyl dihydrojasmonate0.55g
methyl dihydrojasmonate and anhydrous methanol (20ml) are joined in the
three-necked bottle, slowly drip thionyl chloride (SOCl down at 0~-15 ℃
2) (1.5ml), stirring reaction recession in 1 hour deicing salt bath at
room temperature continues to stir after 3~5 hours, boils off anhydrous
methanol on Rotary Evaporators, obtains the target product methyl
dihydrojasmonate.Productive rate 84%, its reaction formula is as
follows:
The production spectra diagram data is as follows:
IR(KBr)v maxcm -1:2955,2930,2859,1739,1437,1166;
1HNMR(400MHz,CDCl 3):δ=0.87(t,J=7.0Hz,3H),1.22-2.65(m,16H),3.71(s,1H);
13CNMR(100Hz,CDCl 3):δ=
219.7,172.6,54.1,51.6,38.9,38.0,37.7,32.0,27.8,27.2,26.3,22.4,14.0。
Use other catalyzer, lewis acidic embodiment same as the previously described embodiments.
*******************************************************************************
Bio-based methyl dihydrojasmonate, bio-based cyclopentanone, preparation method …
WO CN
CN118871415A 阎震 法国特种经营公司
Priority 2022-01-31 • Filed 2023-01-31 • Published 2024-10-29 (Rhodia patent)
The
invention relates to biobased methyl dihydrojasmonate, biobased
cyclopentanone, a preparation method and application thereof.
Background
Methyl
dihydrojasmonate (CAS 24851-98-7) is an aromatic compound, the odor of
which is floral and citrus when in the form of a racemic mixture. The
compounds are used in the perfume industry and the food industry.
Methyl dihydrojasmonate is used as a synthetic equivalent of methyl
jasmonate, a component of naturally occurring jasmine. Industrially,
methyl dihydrojasmonate can be prepared from adipic acid as a precursor
for the preparation of cyclopentanone. Cyclopentanone can then be
functionalized by aldol condensation with valeraldehyde followed by
michael addition of dimethyl malonate.
Synthetic flavors are less
preferred by consumers than natural-derived flavors. Thus, there is
increasing interest in other sources of methyl dihydrojasmonate, and in
particular in ways that use natural raw materials that can be marked as
natural or biological in accordance with existing regulations.
E
xamplesExample 1: preparation of biobased cyclopentanone3G
of biobased furfuryl alcohol (100% biobased carbon content) was
hydrogenated in 60mL of water in the presence of a nickel catalyst (30
mg). The hydrogen pressure was 30 bar and the temperature was 160 ℃.After
stirring for 3-4 hours, the reaction mixture was analyzed. Biobased
cyclopentanone was obtained and showed a biobased carbon content of
100%. The conversion rate is 100%.Example 2: preparation of methyl biobased dihydrojasmonate.The
biobased cyclopentanone of example 1 (100% biobased carbon content) was
reacted with valeraldehyde to produce the compound of formula (III).The compound of formula (III) is then reacted with 100% dimethyl biobased malonate having a 100% biobased carbon content.Methyl dihydrojasmonate was obtained and showed a biobased carbon content of 62%.
Publication number
Priority date
Publication date
Assignee
Title
CH382731A
1960-02-25
1964-10-15
Firmenich & Cie
Process for the preparation of alicyclic keto esters
US4260830A
1980-01-18
1981-04-07
International Flavors & Fragrances Inc.
Process for the preparation of methyl dihydrojasmonate and lower alkyl homologues
DE69031954T2
*
1989-05-23
1998-09-03
Nippon Zeon Co
Fragrance composition
KR101078629B1
*
2005-06-30
2011-11-01
아사히 가세이 케미칼즈 가부시키가이샤
Process for production of substituted cyclopentanone
CN105330523A
*
2015-10-22
2016-02-17
复旦大学
Method for preparing cyclopentanone by taking biomass resource as raw material
CN108863738B
*
2017-05-08
2021-09-07
万华化学集团股份有限公司
Method for preparing cyclopentanone
CN108380206B
*
2018-02-22
2021-04-20
万华化学集团股份有限公司
Method for preparing cyclopentanone through furfuryl alcohol rearrangement hydrogenation
EP4022056A4
2019-08-30
2024-07-03
Lygos Inc
Recombinant host cells for the production of malonate
CN112194577A
*
2020-09-03
2021-01-08
大连理工大学
Method
for preparing cyclopentanone compounds from furfural and furfural
derivatives through aqueous phase hydrogenation rearrangement
* Cited by examiner, † Cited by third party
********************************************************a exploer
CH382731A 1960-02-25 1964-10-15 Firmenich &
Cie Process for the preparation of alicyclic keto
esters starting patent for methyldihydrojasmonate FR US3158644A
* 1960-02-25 1964-11-24 Firmenich & Cie
Alicyclic ketoesters and process for their manufacture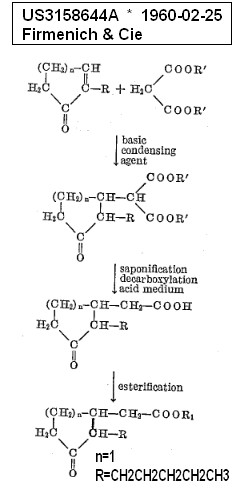
**********************************************************
aldol condensation cyclo +valeraldehyde
info here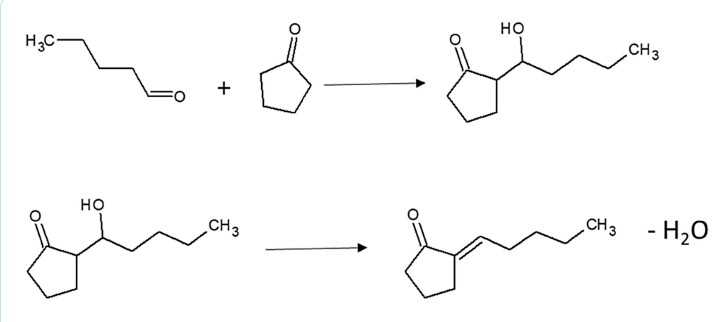
*******************************************************************
US-3978108-A
Cis methyl dihydrojasmonate S.A. Des Etablissements
Roure-Bertrand Fils & Justin Dupont
1970-12-23
Cis methyl dihydrojasmonate
Abstract
Methyl
dihydrojasmonate containing a major proportion of cis methyl
dihydrojasmonate. The NMR spectrum of cis methyl dihydrojasmonate is
illustrated in the attached drawings.Cis methyl dihydrojasmonate
displays olfactory properties superior to those of the trans isomer
making it very useful as an odoriferous agent. In order to prepare
methyl dihydrojasmonate containing a major proportion and preferably at
least 90% of cis isomer, methyl
(2-pentyl-3-keto-cyclopenten-1-yl)-acetate is catalytically
hydrogenated in the presence of an aluminium derivative
The preparation of methyl
dihydrojasmonate, the chemical name of which is methyl
(2-pentyl-3-keto-cyclopentyl)-acetate, is described in as well as in
French Pat. No. 1,280,432 U.S. Pat. No. 3,158,644.
While methyl dihydrojasmonate is capable of existing in both the trans
and cis forms, and is so generally indicated, for instance, in said
U.S. patent, the face is that the cis form has not heretofore been
isolated nor characterized. Furthermore, the process disclosed in said
patents, production of either 100% of the trans isomer or possibly a
very small percentage at best of the cis isomer intermingled with the
essentially overwhelming content of the trans isomer, the latter
constituting no less than about 95% by weight, perhaps more, of the
methyl dihydrojasmonate. These two forms can be represented, in
accordance with the usual conventions, by formulae I and II
respectively. ##SPC1##
A process has now been found which
enables the production of methyl dihydrojasmonate containing a major
proportion, that is, more than 50% by weight, of the cis isomer. The
process of this invention has proved to be capable of giving a methyl
dihydrojasmonate containing upwards of 90% of the cis isomer.
It has
furthermore surprisingly been found that cis methyl dihydrojasmonate
and also methyl dihydrojasmonate containing a major proportion of the
cis isomer is distinguished from the earlier trans product both by its
olfactory characteristics and in that the physicochemical properties of
the cis isomer differ from those of the trans isomer.
Publication number
Priority date
Publication date
Assignee
Title
US3158644A
*
1960-02-25
1964-11-24
Firmenich & Cie
Alicyclic ketoesters and process for their manufacture
DE2008878A1
*
1969-03-10
1970-09-24
L. Givaudan & Cie S.A., Vernier-Genf (Schweiz)
New cycloalkenone esters
GB1206981A
*
1967-10-13
1970-09-30
Toray Industries
Method of activating raney alloys
FR1280432A
*
1961-02-17
1961-12-29
Firmenich & Cie
Alicyclic ketoesters and process for their preparation
****************************************************************************************
US4260830A
1980-01-18
1981-04-07
International Flavors & Fragrances Inc.
Process for the preparation of methyl dihydrojasmonate and lower alkyl homologues
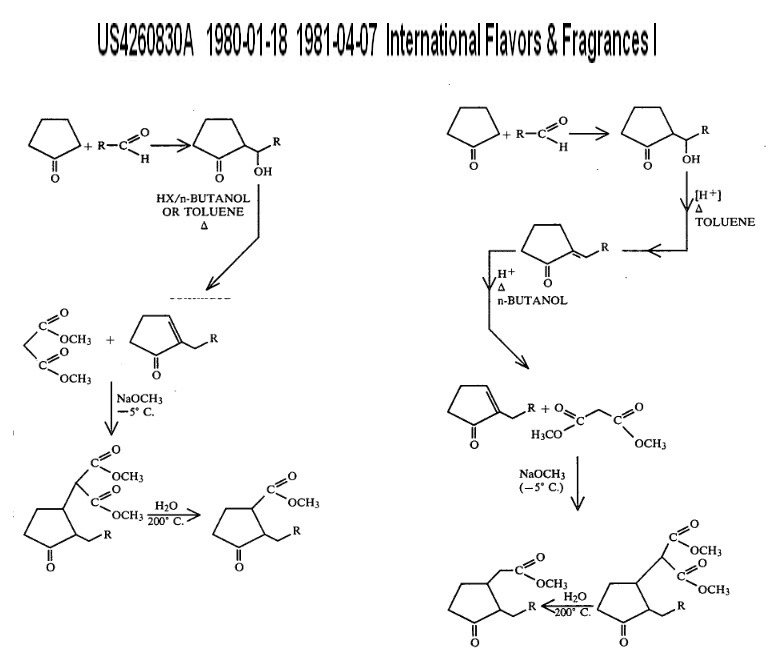 full details here
full details here
****************************************************************************************
more patent litterature references





 full details here
full details here