
| CN108602749B Current Assignee J Cheil Jedang Corp (China) 2015 1. A method of preparing ferulic acid comprising: (a) reacting corn bran with an alkaline solution to obtain a crude extract containing ferulic acid; (b) removing starch from the crude extract obtained in step (a); wherein the starch removal is performed by an enzymatic reaction, wherein the crude extract is reacted with an alpha-amylase, a glucoamylase, or both an alpha-amylase and a glucoamylase; and (c) washing the extracted residue of corn bran. 2. The method of claim 1, wherein the alkali solution is sodium hydroxide or potassium hydroxide. 3. The method of claim 2, wherein the concentration of the alkali solution is 0.5% (w/w) to 1.5% (w/w). 4. The method of claim 1, wherein the reaction is carried out for 1 to 24 hours. 5. The method of claim 1, wherein the solid to liquid ratio between the corn bran and the alkali solution is in the range of 1: 3 to 1:15, or more. 6. The method of claim 1, wherein the reaction is carried out at 60 ℃ to 100 ℃. 7. The method of claim 1, wherein step (a) further comprises filtering the crude extract obtained therein to remove solids. 8. The method of claim 1, wherein the concentration of glucoamylase and alpha-amylase is 0.1% (w/w) to 1.0% (w/w). 9. The method of claim 1, wherein the residue is washed with 1-10 volumes of water compared to the corn bran. 10. The method of claim 1, further comprising (d) isolating and purifying ferulic acid. 11. The method of claim 10, wherein the separating and purifying comprises a primary purification process using activated carbon and a secondary purification process using an adsorbent resin. 12. The method of claim 11, wherein the activated carbon is granular activated carbon or powdered activated carbon. 13. The method of claim 11, wherein the content of activated carbon is 0.1% (w/v) to 2% (w/v) with respect to the concentration of the extract. 14. The method of claim 11, wherein the primary purification process comprises (i) activated carbon adsorption, (ii) hot water washing of the activated carbon, and (iii) desorption of ferulic acid from the activated carbon. 15. The method of claim 14, wherein the desorption is performed using a basic solvent. 16. The method of claim 15, wherein the alkaline solvent is sodium hydroxide or potassium hydroxide. 17. The method of claim 15, wherein the concentration of the alkaline solvent i s 0.01% (w/w) to 0.5% (w/w). 18. The method of claim 14, wherein the primary purification process further comprises adjusting the pH of the treatment solution separated by ferulic acid desorption to 3 to 4 to remove impurities. 19. The method of claim 11, wherein the adsorbent resin is selected from the group consisting of PAD900, Mn100, HP20, and PAD 600. 20. The method of claim 11, wherein the secondary purification process comprises adsorbing the treatment solution obtained in the primary purification process onto an adsorbent resin and desorbing the treatment solution therefrom. 21. The method of claim 11, wherein the desorption is performed with 15% (w/w) to 35% (w/w) ethanol solvent. 22. The method of claim 10, further comprising crystallizing the isolated and purified ferulic acid. more details | 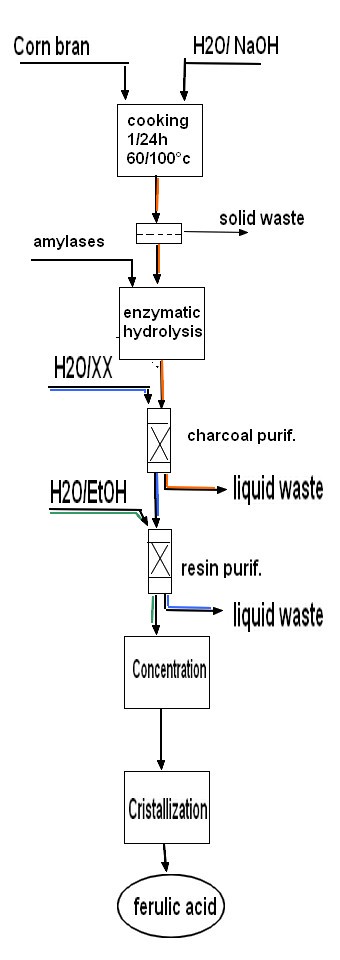 |
| Procedure Rice bran was pulverized 60 mesh sieves, get 400kg rice bran input extractor, adding 3000LpH is 11 calcium hydroxide aqueous solution and 300g vitamins C, and the heating dynamic soaking extracted 1 hour, extracted twice, merged filtrate twice, got 5500L filtered liquid and filter residue. Filtered liquid is passed through 724 type Zeo-karbs, collecting down, fluid injection concentrates, with volume ratio is 5: 4: 8: normal hexane-ethyl acetate, alcohol and water of 1 is miscible in separating funnel, shake up the back standing demix, getting on it is stationary phase mutually, following phase place moving phase, upper and lower phase mixed solution 40ml dissolving concentrated solution 200mg with 1: 1 separates, collect the forulic acid component peaks, lyophilize obtains the forulic acid solids content more than 98%. Filter residue adds the aqueous solution of the pH3 that 2200L mixes up with hydrochloric acid, soaked 4 hours, per 30 minutes ultrasonic 6 minutes, filter, in filtrate, add 154kg sodium-chlor again, stir while adding, stirred 30 minutes, filter, filtrate adds the D202 macroporous anion exchange resin exchange of handling well earlier, make down fluid injection pH reach 7, following fluid injection adds the D011 Zeo-karb again, makes down fluid injection pH reach 2, following fluid injection adds the S-8 macroporous adsorbent resin again, with 95% ethanol elution of 8 times of column volumes, collect elutriant, elutriant is decoloured by the D3520 macroporous resin, destainer concentrates through reduced vacuum, reclaim ethanol, the concentrated solution cryodrying promptly gets the phytic acid product, phytic acid content 88.1%. | 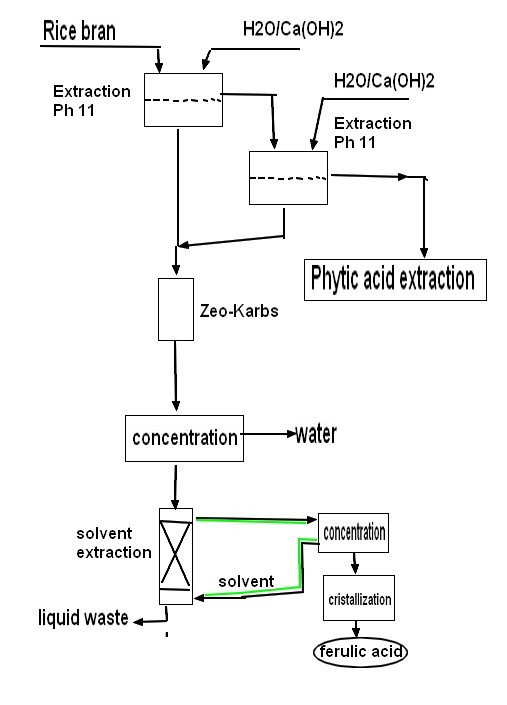 |
This
application describes a process for extracting ferulate (ferulic acid
esters of the variety methyl-, ethyl-, propyl-, butyl-, or any
variation thereof) and coumarate from agricultural biomass such as
miscanthus and corn byproducts. | 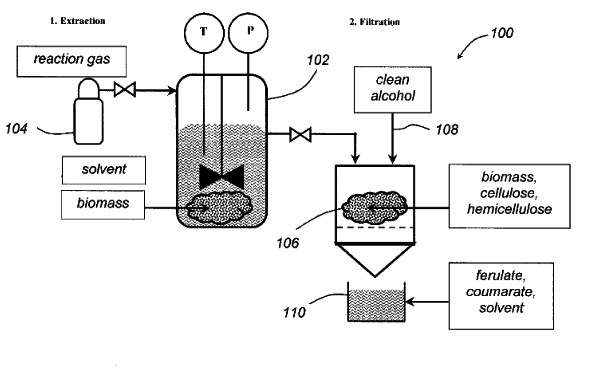 |
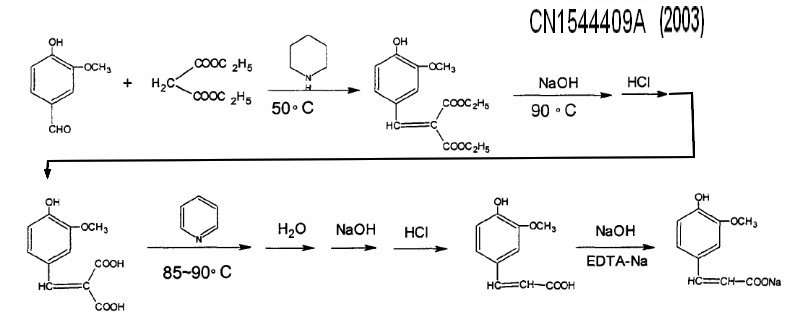 |
The invention discloses a process for preparing ferulic acid by using vanillin and ethyl malonate compounds as synthetic raw material through the steps of condensation, saponification and decarboxylation, wherein the reaction between ferulic acid with suitable alkali metal salt can be carried out to obtain corresponding ferulate, e.g. sodium ferulate. CN1544409A (2003) Assignee Limin Pharmaceutical Factory of Livzon Pharmaceutical Group |
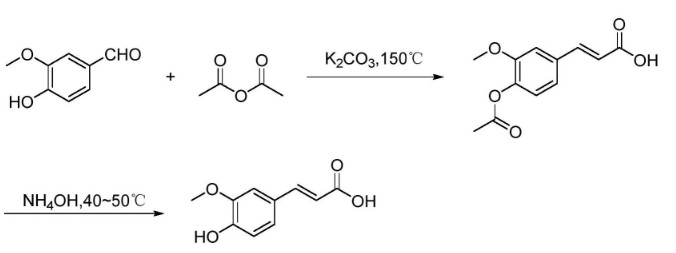 |
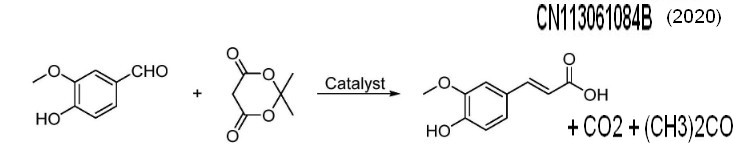
The vanillin is used as a raw material, : the vanillin reacts with acetic anhydride (Perkin reaction) at high temperature in the presence of potassium carbonate to generate acetylferulic acid, and the acetylferulic acid is hydrolyzed under alkaline conditions to obtain the ferulic acid. The synthetic route still uses vanillin as a starting material, is not complex, but has some disadvantages: (1) The reaction temperature is high, and the long-time high-temperature reaction easily causes cis-conformation isomerization of the ferulic acid. (2) The reaction yield is low, the yield of the ferulic acid synthesis step is about 60%, CN105566101a (2015) Current Assignee Shaanxi High Tech Energy Development Co Ltd |
 |
Example 1 |
| Ferulic
acid is a naturally occurring compound found in the cell walls of
plants, particularly in grains like rice, wheat, and oats, as well as
in fruits, vegetables, and coffee. It has gained attention for its
potential health benefits and is widely used in various industries.
Here are some of the key uses of ferulic acid: 1. Antioxidant Properties Ferulic acid is a potent antioxidant that helps neutralize free radicals, reducing oxidative stress and preventing cellular damage. This property makes it beneficial in combating aging and chronic diseases. 2. Skincare In cosmetics and skincare products, ferulic acid is often combined with vitamins C and E to enhance its antioxidant effects. It helps protect the skin from UV radiation, reduces signs of aging (like wrinkles and fine lines), and improves skin texture and tone. 3. Anti-Inflammatory Effects Ferulic acid has anti-inflammatory properties, making it useful in managing inflammatory conditions such as arthritis, cardiovascular diseases, and certain skin disorders. 4. Neuroprotective Effects Research suggests that ferulic acid may protect against neurodegenerative diseases like Alzheimer's and Parkinson's by reducing oxidative stress and inflammation in the brain. 5. Cardiovascular Health It may help improve heart health by reducing cholesterol levels, preventing the oxidation of LDL (bad cholesterol), and improving blood vessel function. 6. Anticancer Potential Ferulic acid has been studied for its potential to inhibit the growth of cancer cells and induce apoptosis (programmed cell death) in certain types of cancer, such as breast, liver, and colon cancer. | 7. Antimicrobial Activity It exhibits antimicrobial properties, making it effective against certain bacteria and fungi. This has potential applications in food preservation and treating infections. 8. Diabetes Management Ferulic acid may help regulate blood sugar levels and improve insulin sensitivity, making it beneficial for individuals with diabetes or at risk of developing the condition. 9. Food Preservation Due to its antioxidant and antimicrobial properties, ferulic acid is used as a natural preservative in the food industry to extend the shelf life of products. 10. Agriculture In agriculture, ferulic acid is used to enhance plant growth and protect crops from oxidative stress and pathogens. 11. Supplements Ferulic acid is available as a dietary supplement, often marketed for its antioxidant and anti-aging benefits. 12. Photoprotection It is used in sunscreens and other photoprotective products to shield the skin from harmful UV rays and prevent sun damage. 13. Wound Healing Ferulic acid has been shown to promote wound healing by reducing inflammation and stimulating tissue repair. 14. Liver Protection Studies suggest that ferulic acid may protect the liver from damage caused by toxins, alcohol, or oxidative stress. 15. Bone Health It may support bone health by reducing bone loss and improving bone density, potentially benefiting individuals with osteoporosis. |
| The
piperazine ferulate can be prepared by taking ferulate and
piperazine hexahydrate as raw materials for reaction. Piperazine
ferulate is the main active ingredient of the traditional Chinese
medicine ligusticum wallichii, has the functions of anticoagulation,
platelet aggregation prevention, microcirculation improvement,
vasospasm relief and coronary artery flow increase, and is clinically
suitable for the auxiliary treatment of glomerulonephropathy
accompanied with microscopic haematuria and hypercoagulable state,
coronary heart disease, cerebral infarction, vasculitis and the like. |
| DE19532317A1
1995-09-01 Haarmann & Reimer Gmbh Process for the production
of vanillin and suitable microorganisms from ferulic acid Claims (4) translated from German 1. Amycolatopsis sp. from the genus Pseudonocardia with the in the German Collection for Microorganisms and Cell Cultures GmbH in Braunschweig under the numbers DSM 9991 and DSM 9992 Tribes. 2. Process for the preparation of vanillin from ferulic acid in the presence of Amycolatopsis sp. DSM 9991 or DSM 9992 or its enzymes or of microorganisms with genetic material from Amycolatopsis sp. DSM 9991 or DSM 9992, which are the structural and regulatory genes for the Encodes enzymes that are effective in this reaction. 3. The method according to claim 2, according to which natural as the starting component uses ferulic acid. 4. Use of the produced by the method according to claims 2 and 3 Vanillins for the production of flavors. | Example 3 Production of vanillin in a 10 l fermenter 5 l culture medium (4 g / l glucose, 10 g / l malt extract and 6 g / l yeast extract) were sterilized in a fermenter and after cooling with 100 ml of a Pre-culture of DSM 9992 inoculated according to Example 1. The culture conditions were: 37 ° C, 500 rpm, 5 l air / min. 12.5 hours after 1.634 kg of an approximately 3.7% ferulic acid solution (60.2 g Ferulic acid) added. After 17.5 hours over a period of 10 Hours another 4.377 kg of an approximately 3.7% ferulic acid solution (164.72 g Ferula acid) pumped in. The fermentation was stopped after 32 hours. The concentration on Vanillin was 11.5 g / l and 1 g / l of unreacted ferulic acid was still present. The final volume was 11.29 l. This is an implementation of 77.8% of theory based on converted ferulic acid. more info here |