| Properties Chemical formula C5H8O Molar mass 84.12 g/mol Appearance clear, colorless liquid Odor peppermint-like Density 0.95 g/cm3, liquid Melting point −58.2 °C Boiling point 130.6 °C /760 Torr Solubility in water Slightly soluble | 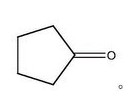 |
The primary synthetic methods of current cyclopentanone is by pyrolysis of hexanodioic acid(adipic acid also mainly produced for NYLON6-6) and cyclopentene oxidizing, but its raw material hexanodioic acid and cyclopentenes derive from fossil energy, and severe reaction conditions.
It is produced by several chemical manufacturers globally.
Some Key Producers of Cyclopentanone:
BASF SE (E.C.)
Rhone Poulenc/RhodiaSA/Syensqo (E.C.)
Zeon Corporation (Japan)
Sinopec (China Petroleum & Chemical Corporation) (China)
Zhejiang NHU Co., Ltd. (China)
- Alkali Metals Ltd. (India)
Patent activity of some Key producers
BASF
BASF patents
| CY-1115779-T1 | METHOD FOR PREPARING CYCLOPENTANONE | 2004-09-23 |
| CY-1115779-T2 | Process for the preparation of cyclopentanone | 2007-06-04 |
| JP-2013502398-A | 4-pentenoic acid production method | 2009-08-21 |
| US-6344580-B1 | Process for the preparation of 2,2-dimethyl-5-(4-chlorobenzyl) cyclopentanone and an intermediate useful therefore | 2000-06-12 |
| EP-1601638-A1 | Method for producing and purifying cyclopentanone | 2003-02-26 |
| DE-3916140-A1 | Substd. cyclopentanone or formyl-cyclopentanone derivs. prodn. | 1989-05-18 |
| DE-3916139-A1 | Substd. cyclopentanone or formyl-cyclopentanone derivs. prodn. | 1989-05-18 |
| DE-3916138-A1 | Substd. cyclopentanone or formyl-cyclopentanone derivs. prodn. | 1989-05-18 |
| EP-0266687-A2 | Process for the manufacture of cyclopentanone | 1986-11-07 |
| DE-3878356-D1 | METHOD FOR PRODUCING CYCLOPENTANONE. | 1987-09-09 |
| US-6316659-B1 | Method for producing cyclopentanone and cyclopentene-1-carboxylic acid and their esters | 1997-09-09 |
| EP-1084096-A1 | Method for producing cyclopentanone | 1998-05-28 |
| DE-10308489-A1 | Process for the preparation of cyclopentanone and caprolactone from dicarboxylic acid solution | 2003-02-26 |
| DE-10319489-A1 | Process for the preparation of cyclopentanone | 2003-04-30 |
| JP-S61218548-A | Manufacture of 2-alkyl-cyclopentanone | 1985-03-09 |
| JP-H037242-A | Production of cyclopentanone | 1989-05-18 |
| US-2007244272-A1 | Method for Separating Ammonia and Water From Mixtures, Arising During The Production of Polyamides | 2004-06-02 |
| GB-999851-A | Process for the conversion of cyclic ketoximes | 1961-06-10 |
| GB-1112397-A | Processing mixtures containing acetic acid | 1964-09-11 |
| GB-728522-A | Improvements in the production of pure adiponitrile | 1951-10-13 |
| US-2007167318-A1 | Catalytically active composition and the use thereof in dehydration methods | 2004-01-30 |
| US-3301891-A | Production of cycloaliphatic nitrates | 1956-02-24 |
| GB-830630-A | Improvements in the production of lactams from oxime hydrochlorides | 1956-06-02 |
| GB-714220-A | Process for exploiting the by-products from the manufacture of nitrogenous compounds having an open or cyclic chain of six saturated carbon atoms | 1951-08-04 |
| GB-788436-A | Improvements in the production of cycloaliphatic ketoximes and their hydrochlorides | 1955-08-05 |
| GB-1396985-A | Process for the production of aliphatic or cycloaliphatic secondary or tertiary amines | 1971-04-15 |
| GB-1390465-A | Production of alpha,beta-unsaturated ketones | 1971-10-13 |
| GB-738888-A | Improvements in the production of oximes | 1952-06-21 |
| GB-1048074-A | Production of lactams by rearrangement of cyclic ketoximes | 1962-07-25 |
| GB-888501-A | Production of bicyclo-[2,2,1]-heptadiene-(2,5) | 1959-08-13 |
| DE-3878356-D1 | METHOD FOR PRODUCING CYCLOPENTANONE. | 1987-09-09 |
| DE-3916139-A1 | Substd. cyclopentanone or formyl-cyclopentanone derivs. prodn. | 1989-05-18 |
| DE-3916138-A1 | Substd. cyclopentanone or formyl-cyclopentanone derivs. prodn. | 1989-05-18 |
| DE-3916140-A1 | Substd. cyclopentanone or formyl-cyclopentanone derivs. prodn. | 1989-05-18 |
| DE-3771657-D1 | METHOD FOR PRODUCING CYCLOPENTANONE. | 1986-11-07 |
Zeon Corporation
Zeon patents
| JP-2012219060-A | Method of producing cyclopentanone | 2011-04-08 |
| JP-2017178824-A | Method for producing cyclopentanone | 2016-03-29 |
| JP-2007191445-A | Apparatus and method continuously producing cyclic ketone compound | 2006-01-20 |
| EP-0016650-A2 | 2-Cyclopentyl-cyclopentanone, fragrance or flavouring compositions containing it and their use | 1979-03-26 |
| JP-S597129-A | Preparation of cyclopentanone | 1982-07-05 |
| JP-S58203932-A | Production of cyclopentanone | 1982-05-21 |
| JP-S58150534-A | Preparation of cyclopentanone | 1982-03-03 |
| JP-2004131439-A | Method for producing 2,5-disubstituted cyclopentanone compound and 2,5-disubstituted cyclopentanol compound | 2002-10-11 |
| JP-2008088071-A | Method for producing 2-cyclopentyl cyclopentanone compound | 2006-09-29 |
| JP-H02306929-A | Production of 2,3-disubstituted cyclopentanone mixture | 1989-05-23 |
| JP-2001335527-A | Method for producing cyclopentanone | 2000-05-30 |
| JP-H1072399-A | Production of 2-substituted cyclopentanone | 1996-08-30 |
| JP-2003040821-A | 2,5-disubstituted cyclopentanone and perfume composition | 2001-07-30 |
| JP-2020169202-A | Method for producing cyclopentanone | 2020-07-06 |
| EP-0612715-A2 | Fragrant composition | 1989-05-23 |
| US-5372994-A | Fragrant composition | 1989-05-23 |
| JP-S604179-A | Production of delta-lactone | 1983-06-20 |
| JP-S604180-A | Production of delta-substituted-delta-lactone | 1983-06-20 |
| JP-S57197275-A | Production of alpha-lactone | 1981-05-29 |
| JP-S57209285-A | Preparation of delta-lactone | 1981-06-17 |
| JP-S57209284-A | Preparation of delta-lactone | 1981-06-17 |
| JP-S58170777-A | Preparation of 8-lactone | 1982-03-31 |
| JP-2004083446-A | Equilibrium mixture, its manufacturing method and method for manufacturing 1-alkoxy-2-oxabicyclooctane compound | 2002-08-23 |
| JP-2000119691-A | Detergent and cleaning method using the same | 1998-10-12 |
| JP-H01171496-A | Production of 9alpha-hydroxysteroid | 1987-12-25 |
| JP-2000119693-A | Detergent and cleaning method using the same | 1998-10-12 |
| JP-H06179635-A | Production of steroid intermediate | 1993-07-19 |
| JP-H08262711-A | Positive type resist composition | 1995-03-24 |
| JP-S5756401-A | Wood preservative | 1980-09-19 |
| JP-S5748931-A | Preparation of carbonyl compound | 1980-09-09 |
| JP-S5748934-A | Preparation of 2-substituted cycloalkanone | 1980-09-10 |
| JP-S5980619-A | Oxidation of cyclopentene | 1982-10-29 |
| JP-2002121394-A | Curable composition | 2000-10-13 |
| JP-H10123725-A | Developer for photosensitive polyimide | 1996-10-18 |
| JP-2001261609-A | High-purity cyclopentane derivative having oxygen- containing group, method for producing the same and perfumery composition containing the same | 2000-03-22 |
| JP-2019133104-A | Positive resist composition, method for forming resist film, and method for manufacturing laminate | 2018-02-02 |
| JP-S55120533-A | Preparation of 2-alkyl-2-cyclopentenone | 1979-03-09 |
| JP-S61122244-A | Production of cycloalkanecarboxylic acid compound | 1984-11-19 |
| JP-2008228804-A | Deodorant and fiber product using the deodorant | 2007-03-16 |
| JP-2004137177-A | Method for producing 2-oxocyclopentane carboxylate | 2002-10-16 |
| JP-2004083447-A | 2-oxabicyclo[3.3.0]octane compound, its manufacturing method and optical resolution agent | 2002-08-23 |
| WO-0022081-A1 | Heptafluorocyclopentane composition, method of cleansing, and method of recovery | 1998-10-09 |
| JP-H05271142-A | Production of carbonyl compound | 1992-03-27 |
Rhone Poulenc/RhodiaSA/Syensqo
| US-5600013-A | Preparation of cyclic ketones | Rhone-Poulenc Chimie | 1993-05-28 |
| US-5856581-A | Preparation of cyclic ketones | Rhone-Poulenc Chimie | 1993-05-28 |
| DE-69403882-D1 | Process for the C-alkylation of a ketonic compound | Rhone Poulenc Chimie | 1993-11-22 |
| EP-0626364-A1 | Process for the preparation of a cyclic ketone | Rhone-Poulenc Chimie | 1993-05-28 |
| EP-0722432-A1 | Method for preparing of mono or di-2-substituted cyclopentanone | Rhone-Poulenc Agrochimie | 1993-10-07 |
Sumitomo
| JP-S57128649-A | Cyclopentanone derivative | 1981-01-30 |
| JP-S60224656-A | Production of cyclopentanone derivative | 1984-04-20 |
| JP-S6092234-A | Production of cyclopentanone | 1983-10-26 |
| JP-S57188540-A | Preparation of cyclopentanone compound | 1981-05-13 |
| JP-H07118693-A | Cleaning solution for sensitizing agent production device, cleaning method using the same, cleaning solution for resist solution preparation device and cleaning method using the same solution | 1993-08-30 |
| JP-H04149153-A | Cyclopentanealdehydes and production thereof | 1990-10-12 |
| JP-2004168774-A | Method for producing 2-bromocyclopentanone | 2002-11-08 |
| JP-S6064943-A | Production of optically active 4-hydroxycyclopentenone | 1983-08-10 |
| JP-S59187788-A | Method for separating cyclopentenone | 1983-04-08 |
| JP-2004109423-A | Color photosensitive resin composition | 2002-09-18 |
| JP-S6137756-A | Production of cyclopentylacetic acid | 1984-07-30 |
| JP-H06320128-A | Washing solution and method for spin coater line piping | 1993-05-18 |
| JP-S61106537-A | Production of cyclopentylacetic acid ester | 1984-10-30 |
| JP-H0665133-A | Production of optically active 4-hydroxy-2-cyclopentenone | 1992-08-20 |
| JP-S62223149-A | Production of optically active 4-hydroxycyclopentenone | 1986-03-25 |
| JP-H08143542-A | Unsaturated aldehyde derivative, intermediate, their production and use | 1994-11-24 |
Last worldwide patents for 2020 to 2024 Period
Total 195 patents mostly from China corporation and /or Universities (90% from Chnia , 10% rest of world)
All details : patents2020_2024
CYCLOPENTANONE SYNTHESIS
Cyclopentanone from adipic acid
Adipic acid is widely available from various source , being one of the raw material used to produce NYLON 6-6 by reaction with hexamethylene diamine .The production of cyclopentanone is generally done in continuous with a proprietary catalyst at temperature in the 250/300° c range depending on the catalyst used . The cyclopentanone chemical yield is over 95% , the purification is done by distillation | 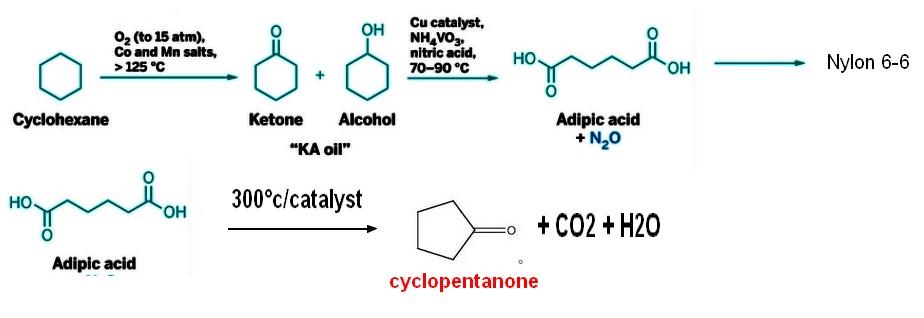 |
Cyclopentanone from cyclopentene
Cyclopentene (liquid boiling point 45°c/760 torr) is produced industrially in large amounts by steam cracking of naphtha.
By oxydation it can lead to cyclopentanone using various catalyst and solvants .
Here some examples of patents taken by different chemical corporations around the world
| Oxydation with hydrogene peroxide (Shandong Chambroad Petrochemicals Co procedure 2024 ) The process consist of oxidizing cyclopentene with hydrogen peroxide aqueous solution, in presence of a specific catalyst, generating cyclopentanol by water addition on cyclopentene under the action of an acid center, The cyclpentanol generated is oxydized into cyclopentanone under the action of hydrogen peroxide and a titanium silicon molecular sieve, This is a one-step method thanks to the action of a modified molecular sieve; the production method has the advantages of simple operation, mild condition, high selectivity of cyclopentanone (.CN118666655A Worldwide applications 2024 CN Shandong Chambroad Petrochemicals Co Ltd claiming 50% conversion of cyclopentene into cyclopentanone with 99% selectivity ) lab_experimental procedure | 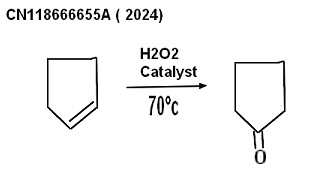 |
Oxydation with hydrogene peroxide Nippon Zeon recipe 1982 JP-S5980619-A Oxidation of cyclopentene Nippon Zeon Co Ltd 1982-10-29 Abstract To obtain an oxygen-containing compound useful as a raw material of perfumes, pharmaceuticals, agricultural chemicals, etc., continuously, in high yield, by oxidizing cyclopentene in the presence of a palladium compound and a molybdenum-based heteropolyacid compound in combination with hydrogen peroxide. CONSTITUTION:An oxygen-containing compound such as 2-cyclopenten-1-one, cyclopentanone, cyclopentanol, etc. is prepared economically, in an industrial scale, without loss of the catalytic activity, by oxidizing cyclopentene in the presence of (A) a palladium compound, (B) a molybdenum-based heteropolyacid compound and (C) hydrogen peroxide, at 20-80 deg.C, preferably using a solvent which dissolves at least a part of the components A-C. The molar ratios of A and B to cyclopentene are preferably about 0.1-0.005 and 2-20, respectively, and that of C to palladium is about 2-20, from the economical view point. | 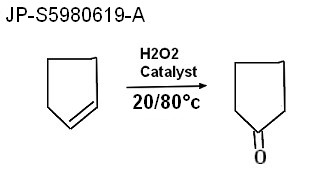 |
| Oxydation with nitrous oxide (N2O) BASF procedure 2007 WO-2008148661-A1 Method for producing cyclopentanone Basf Se 2007-06-04 Abstract The invention relates to a method for producing cyclopentanone. Said method comprises the step of reacting a mixture (G1), which contains at least cyclopentene, with a mixture (G2), which contains at least dinitrogen monoxide. The reaction is carried out in at least one reactor (R1) having channels with a diameter in the range of 0.1 mm to 50 mm, the reactor comprising at least two zones (Z1) and (Z2) having channels with different diameters and the diameters of the channels of zone (Z1) being smaller than the diameter of the channels of zone (Z2). According to a preferred embodiment, the reaction is carried out in the temperature range between 270 and 300 0 C and at a pressure of 260 to 350 bar, in particular 280 bar. The reaction conditions are preferably selected such that the N 2 O conversion is more than 80% and the cyclopentene conversion is more than 50%. Pilot examples in details | 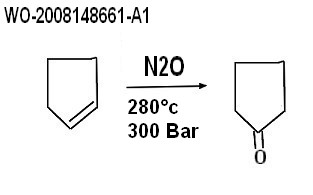 |
| Oxydation with oxygene ( Sumitomo recipe ) JPS6092234A Current Assignee Sumitomo Chemical Co Ltd 1983 Example 1 Cyclopentene of 84, 19 and ethanol of 85.39 were charged into a 200 cc round bottom flask with a cooler, 19.4g bismuth nitrate and 10.6P as catalyst After adding a commercially available 5% PD-activated carbon supported catalyst (manufactured by Nippon Engelhard Co., Ltd.), 99% pure oxygen (the remainder being nitrogen) was continuously supplied at 51/r into the well-stirred liquid. The reaction temperature was set at 45°C, and after 4 hours of reaction, the reaction product was analyzed, and the conversion of cyclopentene was 75%. The selectivity for cyclopentanone was 72%. Comparative Example 1 The same treatment as in Example 1 was carried out except that bismuth nitrate was not added. As a result of analysis of the obtained reaction product, the conversion rate of cyclopentene was 26%, and the selectivity of cyclopentanone was 1% or less. lab_procedure_example (Sumitomo1983) | 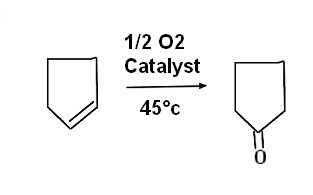 |
| Isomerization of cyclopentene oxide JP-S6330446-A Idemitsu Petrochem Co Ltd, 1986-07-23 Abstract PURPOSE:To obtain the titled compound, which is a raw material for sebacic acid, intermediate raw material for medicines, etc., by bringing cyclopentene oxide into contact with a catalyst carrying palladium in the presence of hydrogen with a long catalyst life without causing corrosion of a reactor. CONSTITUTION:Cyclopentene oxide is isomerized in the presence of a catalyst carrying palladium and hydrogen a 10-100 deg.C, preferably 20-70 deg.C temperature under 0.01-11kg/cm<2>, preferably 1-6kg/cm<2> (absolute pressure) of hydrogen partial pressure to afford the aimed cyclopentanone. The amount of the catalyst used is within the range of 0.001-0.1g atoms, preferably 0.001-0.05g atoms expressed in terms of palladium atoms based on 1mol cyclopentene oxide. | 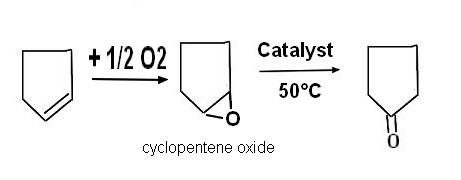 |
More Patents references using cyclopentene as raw material
Cyclopentanone From cyclopentanol
Cyclopentanol can be producded starting from cyclopentene (more info here) and also very often depending on demand from cyclopentanone ! .
CN102603506A current Assignee Paierke chemical materials (Qidong) Co., Ltd. 2012The invention relates to a method for preparing cyclopentanone through cyclopentanol dehydrogenation. The method comprises the steps of carrying out catalytic dehydrogenation and rectification reactions on the raw material cyclopentanol so as to directly obtain the high-purity cyclopentanone, wherein the catalytic dehydrogenation temperature is 85-115 DEG C, the system pressure is 100-300mmHg, and granular Raney nickel type metal alloy comprising 53% of Al, 44% of Ni and 3% of Mo is adopted as a catalyst in the dehydrogenation reaction; and the cyclopentanol load WWH of the catalyst is 2-4hr-1. The gas-phase dehydrogenation reaction product directly enters in a rectifying column of which the number of theoretical plates is 45 to be purified and the reflux ratio of the rectifying column is controlled to be (2:1)-(5:1)to ensure that a cyclopentanone product with the purity of more than 99.9% is obtained. Compared with the prior art, the method disclosed by the invention has the advantages that the energy consumption is low; the use efficiency of the catalyst is high; the production cost is greatly reduced; the purity of the obtained cyclopentanone product is more than 99.9%; and a green and environment-friendly production process is provided. | 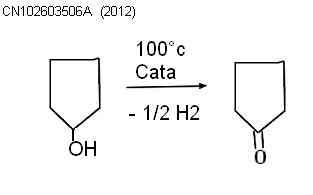 |
More patents using cyclopentanol as raw material
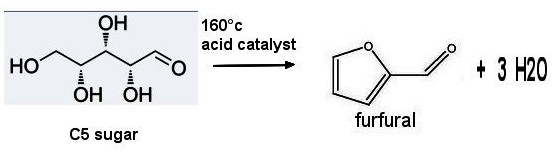
Cyclopentanone From furfural
Furfural is a biomass-derived material, and is industrially produced on a large scale from inexpensive agricultural and forestry wastes (such as corn cobs, bagasse, cottonseed hulls, and the like) as a raw material. The furfural and the cyclopentanone both have five carbon atoms, so that the furfural is directly converted into the cyclopentanone, and the application value is important. The preparation of cyclopentanone by hydrogenation rearrangement is also gradually a hot spot of biomass research especially in China
Furfural is an organic compound derived from agricultural byproducts such as corncobs, oat, wheat bran, and sawdust. It is produced through the acid hydrolysis of pentosans, which are polysaccharides that yield pentoses (five-carbon sugars) like xylose upon hydrolysis. The raw materials are first cleaned and ground to increase the surface area for hydrolysis.The ground material is treated with a dilute acid (usually sulfuric acid) at elevated temperatures (around 150-200°C). This step hydrolyzes the pentosans into pentose sugars, primarily xylose.The pentose sugars undergo dehydration in the presence of the acid catalyst, leading to the formation of furfural. The furfural is then separated from the reaction mixture through steam distillation. The furfural vapor is condensed , collected and purified by distillation .
Firstly furfural is hydrogenated to generate furfuryl alcohol, the furfuryl alcohol is rearranged to generate 4-hydroxy-cyclopent-2-enone (PIANCATELLI rearrangement ),
The 4-hydroxy-cyclopent-2-enone is then subjected to hydroxy dehydration and double bond hydrogenation to generate cyclopentanone,
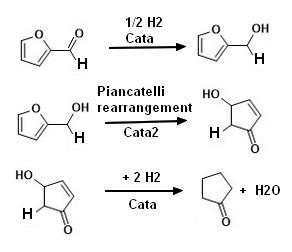 | 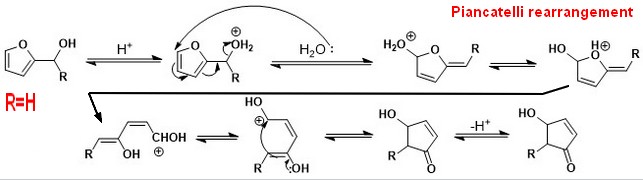 |
The temperature required by PIANCATELLI rearrangement reaction of furfuryl alcohol is high, but the higher temperature can cause side reactions such as hydrogenation of carbon-carbon bonds in the furfural to generate tetrahydrofurfuryl alcohol, methyl furan and even cyclopentanol . Therefore, the process for preparing cyclopentanone by using furfural it is difficult to achieve both the hydrogenation step and PIANCATELLI rearrangement of furfuryl alcohol. In order to reduce the generation of cyclopentanol and other byproducts, the whole reaction temperature must be reduced, but the PIANCATELLI rearrangement reaction rate of furfuryl alcohol is very slow, and in order to improve the rearrangement reaction rate of furfuryl alcohol PIANCATELLI, a large amount of water is required to be added into the reaction system, which causes the concentration of substrate furfural to be too low, thereby resulting in low production efficiency and high subsequent separation cost.
On top of this the intermediate furfuryl alcohol and 4-hydroxy-cyclopent-2-enone are easy to undergo side reactions of polymerization at the reaction temperature, and the polymeric byproduct is sticky t
Achieving high selectivity for cyclopentanone without forming byproducts (e.g., levulinic acid, resins, or polymers) is challenging , Catalyst Stability is also an issue : catalysts may deactivate over time due to fouling or poisoning It should be able to drive both hydrogeanation and PIANCATELLI reaction.
One pot synthesis looks possible , however it looks more promizing to split the various reactions as indicated in Patent CN-115894196-B For the time being productivity and overall yield are modest but a lot a research is underway to improve this situation as it can be seen by the various patents taken recently from China universities , China being , the first world producer for furfural .
Example of lab Procedures
One of latest relevant patent :CN-115894196-B Method for continuously synthesizing cyclopentanone from furfural 2022-11-17 Application filed by Ningxia Xinhua Chemical Co ltd, ZHEJIANG XINHUA CHEMICAL CO Ltd, Zhejiang University ZJU
here many more patents
Some examples of cyclopentanone use
Cyclopentanone is a versatile compound in organic synthesis, and it's used as a raw material in several important processes. Here are a few examples of where cyclopentanone plays a role:
Synthesis of Pharmaceuticals:
Steroid Synthesis: Cyclopentanone is often used as a starting material in the synthesis of steroid compounds, which are used in a wide range of pharmaceutical applications. For example, it can be part of the synthesis of corticosteroids or sex hormones.
Synthesis of Flavor and Fragrance :
It is used in the fragrance industry for the synthesis of certain flavor and fragrance compounds. One example is the production of methyldihydrojasmonate, which is used as a scent in perfumes. (more info on dihydrojasmonate)
Due to its chemical reactivity it can lead to different products used in various sectors as it can be seen looking at the patent litterature
AVIATION FUEL FROM GREEN CHEMISTRY
CN-106867565-A A KIND OF PREPARATION METHOD OF HIGH DENSITY LIQUID HYDROCARBON FUEL 中国科学院大连化学物理研究所 2015-12-12 2017-06-20
Application filed by Dalian Institute of Chemical Physics of CAS
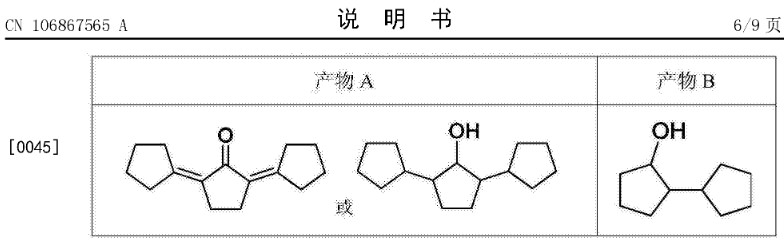
In this patent, we use cyclopentanone as raw material, by a fixation equipped with dual catalyst bed Bed reactor is directly synthesized the high density aviation fuel that main component is bicyclic pentane and three pentamethylene.At first Catalytic bed, cyclopentanone under the catalysis of acid/base catalyst or metal-doped solid base, by itself aldol Condensation reaction (and hydrogenation reaction), obtains the oxygen-containing organic compound that carbon number is 15 and 10;Connect in fixed bed Second beds of continuous formula reactor, carbon number be 15 and 10 oxygen-containing organic compound in carried metal Under the catalysis of A/X type bifunctional catalysts, lower temperature (100-300 DEG C), it is solvent-free under the conditions of enter The step hydrogenation deoxidation of row one, obtains the polycyclic HC fuel of carbon number 15 and 10.Whole process avoid catalyst with The separation of product, realizes chemical industrial integrated, is conducive to the continuous prodution of Future high-density aviation fuel.
JP-H08105827-A Solvent for pathological examination YWako Pure Chem Ind Ltd, 1994-08-09 PURPOSE: To obtain a solvent having volatility equal to that of an organic solvent such as xylene and safety equal to that of a limonene-base solvent
JP-H0577903-A Garbage bag Azetsukusu:Kk, 株式会社アゼツクス 1991-09-24 Abstract
PURPOSE:To provide a garbage bag to which dogs or cats do not come near even when it is left alone. CONSTITUTION:A garbage bag is formed out of a synthetic resin sheet having the weight ratio of 1ppm or more of at least one of menthone, menthol, limonene, alpha-pinene, 3-methylcyclopentanone or cyclopentanone as odor components. Dogs or cats dislike the odor of these components, so that they do not come near to the bag.
JP-H05218092-A Manufacture of field-effect transistor Nec Corp, 日本電気株式会社 1992-01-28
Abstract
PURPOSE:To enhance the breakdown strength, and at the same time, to reduce the capacitance between gate and source by making it difficult to allow the field concentration on the gate electrode. CONSTITUTION:An insulating film 4 and a metallic film 5 are formed on a GaAs substrate in that order. After the metallic film 5 and the insulating film 4 are anisotropically etched with a resist 6 as a mask, an operating layer 3 is etched to form a recess. Then, a resist 7 made of cyclopentanone and 2-(2- methoxy ethoxy) ethanol as the main components is coated in order to deposit a gate metal 8 while leaving resists 7a and 7b in the recess immediately below the insulating film 4 by the irradiation of ultraviolet rays and development.
JP-S5756401-A Wood preservative Nippon Zeon Co Ltd 1980-09-19
Abstract
PURPOSE: An oil-soluble safe wood preservative, containing cyclopentylidenecyclopentanone and cyclopentylcyclopentanone as active constituents, and having improved permeability and operational rate without irritant action and "kabure" on the skin.
CONSTITUTION: A wood preservative containing cyclopentylidenecyclopentanone and cyclopentylcyclopentanone as active constituents. The cyclopentylidenecyclopentanone is generally synthesized by condensing two molecules of cyclopentanone through dehydration, and the cyclopentylcyclopentanone is synthesized by hydrogenating the cyclopentylidenecyclopentanone. Both of them are colorless and transparent liquid substances and have a low viscosity and antimicrobial activity against fungi, e.g. wood destroying fungi or molds, which are microorganisms causing the deterioration of wood. The preservative permeates into the central part of the wood readily to keep the antiseptic (preserving) effect for a long time.
CN-114878557-A Kit for rapidly detecting TNT 北京理工大学 2022-04-29 Kit for rapidly detecting TNT
Abstract
The invention relates to a rapid chemical detection method for common explosive TNT, belonging to the technical field of energetic materials. First by configuring one of the components a of the different detection reagents: KOH-ethanol, benzaldehyde, diphenylamine-concentrated sulfuric acid and cyclopentanone-tetraethyl ammonium hydroxide. The preparation method of the KOH-ethanol solution comprises the following steps: weighing KOH and ethanol according to the mass ratio of 1:20, and uniformly mixing. The preparation method of the diphenylamine-concentrated sulfuric acid comprises the following steps: diphenylamine and concentrated sulfuric acid are respectively weighed according to the mass ratio of 1:200 and then uniformly mixed. The preparation method of cyclopentanone-tetraethylammonium hydroxide comprises the following steps: respectively weighing cyclopentanone, 25% tetraethylammonium hydroxide aqueous solution and ethanol according to the mass ratio of 1:2:12, adding into a beaker, and uniformly mixing. After the preparation is finished, the component A, the ammonium dodecyl sulfonate and a sample to be detected are uniformly mixed, and the TNT can be rapidly detected. The method can quickly detect the existence of TNT, is simple and convenient to operate and is suitable for application of explosion field personnel.
Many more examples of usages here